Have you ever wondered why, despite the vast emptiness of space between atomic particles, objects don’t just pass through each other? At a glance, the notion that solid objects should, under certain conditions, be able to pass through one another might seem reasonable. After all, atoms, the building blocks of matter, are mostly empty space. However, the reality is bound by the laws of physics, particularly those of electromagnetism and quantum mechanics. This article explores the fascinating reasons behind why the physical world behaves as solidly as it does.
Understanding Atomic Structure
The key to understanding why objects don’t pass through each other begins at the atomic level. Atoms consist of a nucleus made up of protons and neutrons, surrounded by a cloud of electrons. Although it’s true that atoms are mostly empty space, they exhibit properties of solidity due to the interactions of these particles.
The Role of Electromagnetic Forces
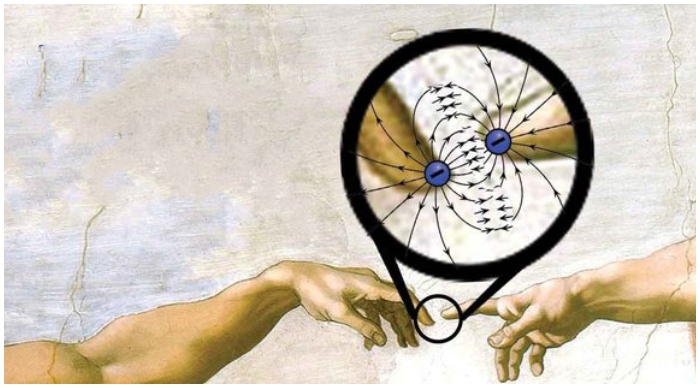
One of the fundamental forces at play is the electromagnetic force, one of the four fundamental interactions in nature. This force involves the interaction between particles that have an electric charge. In atoms, electrons (which are negatively charged) orbit a nucleus made of protons (which are positively charged) and neutrons (which are neutral).
When two atoms come close to each other, the electron clouds interact with each other. The negative charge of the electrons repels other electrons from nearby atoms. This repulsive force is what keeps the atoms from collapsing into each other and what makes materials solid. It is the electromagnetic force that essentially prevents you from passing your hand through a table or walking through walls.
Quantum Mechanics and the Pauli Exclusion Principle
The principles of quantum mechanics, particularly the Pauli Exclusion Principle, also play a crucial role in ensuring that objects do not pass through each other. The Pauli Exclusion Principle states that no two electrons can occupy the same quantum state simultaneously within a quantum system. This principle is what keeps the electrons in atoms spinning in different orbits or levels.
Because of this exclusion, electrons maintain a certain structure in their orbits around the nucleus, which also contributes to the solidity of matter. The structure and spacing dictated by the quantum states of electrons mean that, at a quantum level, there is a repulsion between atoms that prevents them from merging into one another.
Energy and Permeability
Another aspect to consider is the amount of energy that would be required to overcome these forces. To push atoms into each other, an immense amount of energy would be needed, far more than what is typically available in everyday interactions. This is why, under normal circumstances, matter appears and behaves as solid.
Everyday Observations and Misconceptions
The perception that objects are solid and impenetrable is an everyday experience, but it’s fascinating to think that at a microscopic level, forces and principles are continuously at play to maintain this solidity. It’s a common misconception that objects might pass through each other if only they could align perfectly. However, due to the forces and principles discussed, such alignment would not change the fundamental interactions at the atomic level.
Conclusion
The solidity of objects, a quality of matter that defines much of our interaction with the physical world, is dictated by the complex interplay of electromagnetic forces and quantum mechanics. The reasons why objects don’t pass through each other, despite being made up of atoms that are mostly empty space, highlight the fascinating and counterintuitive nature of physics. Understanding these principles not only deepens our appreciation of the physical world but also underscores the remarkable structure and stability of matter as dictated by the laws of the universe.